The Pinometostat (EPZ5676) DOT1L Inhibitor is a novel sub-class of targeted epigenetic therapy that has transformed the concept of leukemia treatment. This compound is more specific and perhaps less toxic to the patient because it does not kill dividing cells indiscriminately but rather by targeting the molecular pathways that promote malignancy.
Pinometostat (EPZ5676) DOT1L Inhibitor is a drug developed by Epizyme Inc. and is a specific histone methyltransferase enzyme that has been implicated in a subgroup of aggressive leukemias.
Let’s unpack the biological rationale, the mechanism of action, clinical evidence, and future potential of Pinometostat (EPZ5676) DOT1L Inhibitor to treat genetically defined cancers.
What Is DOT1L and What is Its role in Leukemia?
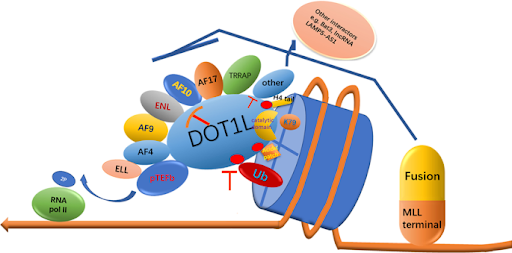
DOT1L ( Disruptor of Telomeric Silencing 1-Like) is an enzyme catalyzing the lysine 79 of histone H3 (H3K79) methylation. This is an important histone modification that regulates transcription of genes, repair of DNA and cell cycle. In a regular setting, DOT1L-mediated methylation promotes correct chromatin structuring and expression of genes.
In some of such leukemias, though, and especially in those that contain Mixed-Lineage Leukemia (MLL) gene rearrangements, DOT1L is aberrantly recruited to oncogenic gene loci. Mutual fusion of MLL with transcriptional co-activators including AF4, AF9, ENL, or AF10 results in the mislocalization of DOT1L and hyper-methylation of H3K79 sites. This irregular methylation enhances the over-expression of leukemia-enhancing genes like HOXA9 and MEIS1 that result in malignant change.
To stop this process, Pinometostat (EPZ5676) DOT1L Inhibitor was developed to selectively inhibit the catalytic activity of DOT1L, subsequently reversing the pathological pattern of gene expression that contributes to the proliferation of leukemic cells. Learn more relevant info here.
How the DOT1L Inhibitor Pinometostat (EPZ5676) Works
One such small-molecule inhibitor of DOT1L’s methyltransferase activity is the Pinometostat (EPZ5676) DOT1L Inhibitor. It stops DOT1L from transferring methyl groups to histone H3K79 by competing with S-adenosylmethionine (SAM) for binding to the enzyme’s active site.
Moreover, the oncogenic transcriptional processes are silenced by Pinometostat (EPZ5676) DOT1L Inhibitor through a series of downstream actions that begin with inhibiting H3K79 methylation. Inhibitor therapy reduced HOXA cluster gene expression, suppressed leukemic proliferation, and induced differentiation in MLL-rearranged cells in preclinical investigations.
The exceptional specificity of Pinometostat (EPZ5676) DOT1L Inhibitor is its defining feature. It doesn’t change methylation marks unrelated to DOT1L and spares other histone methyltransferases. Because of its pinpoint accuracy, it is a potential epigenetic treatment option with minimal off-target effects.
Clinical Assessment and Performance
Pinometostat (EPZ5676) DOT1L Inhibitor individuals with relapsed or refractory acute leukemias with MLL rearrangements were the subjects of the first-in-human clinical trial. Fatigue, nausea, and mild myelosuppression were among the controllable adverse effects of Pinometostat’s continuous intravenous infusion, as shown in the Phase I trial (NCT01684150).
To validate target engagement, pharmacodynamic investigations showed that H3K79 methylation was strongly inhibited in leukemic blasts. Several individuals showed partial responses or illness stability, however the objective response rates were small when used alone. Molecular studies showed that after treatment, MLL fusion-dependent transcriptional targets were downregulated.
You should also know that Pinometostat (EPZ5676) DOT1L Inhibitor was shown to be an effective proof-of-concept therapy for epigenetic dysregulation based on these results. Combination tactics to increase its therapeutic effect are the subject of ongoing research.
Combination Therapies and Synergistic Effects
The complexity of leukemogenic signaling is the primary reason why monotherapy with Pinometostat (EPZ5676) DOT1L Inhibitor shows biologic effectiveness but poor durability. As a result, researchers are looking into ways to increase the therapeutic effects by using combination techniques.
Pinometostat (EPZ5676) DOT1L Inhibitor with traditional chemotherapy drugs like cytarabine and anthracyclines may work together synergistically, according to preclinical evidence. Pinometostat increases apoptosis in leukemic cells by making them more sensitive to DNA-damaging chemicals through reprogramming transcriptional networks.
Combination with BCL-2 inhibitors, such as venetoclax, has also had encouraging results. It is possible to disrupt survival pathways and epigenetic regulation concurrently by co-targeting DOT1L and BCL-2, as MLL-rearranged leukemias display BCL-2 reliance. Combination regimens using Pinometostat (EPZ5676) DOT1L Inhibitor have been the subject of numerous preclinical and early-phase clinical trials as a result of these findings.
Pharmacological Properties and Delivery
The drug’s pharmacokinetic profile was an early concern with Pinometostat (EPZ5676) DOT1L Inhibitor. To keep effective plasma concentrations, the drug has to be continuously infused into the bloodstream due to its short half-life and low oral bioavailability. The development of new analogs and delivery mechanisms that can inhibit DOT1L for an extended period of time after oral or subcutaneous injection is now a focus of research.
Whatever the case may be, the Pinometostat (EPZ5676) DOT1L Inhibitor was an innovative chemical that proved DOT1L may be a therapeutic epigenetic target. Its path through clinical trials has cleared the way for more potent and pharmacodynamically tuned second-generation inhibitors.
Additional Implications for Epigenetic Treatment
Beyond MLL-rearranged leukemia, Pinometostat (EPZ5676) DOT1L Inhibitor has been successful. Some solid cancers, which involve oncogenic transcription due to abnormal histone methylation, have been linked to DOT1L dysregulation. By extension, this chemical lays the groundwork for addressing cancer by targeting epigenetic enzymes associated with transcriptional addiction.
Moreover, drugs that alter gene expression rather than genetic code are becoming increasingly important, and the creation of Pinometostat (EPZ5676) DOT1L Inhibitor is an example of this. With this level of accuracy, disease regulation is possible with less systemic toxicity than with traditional chemotherapy.
Challenges and Future Directions
Lastly, you should know that Pinometostat (EPZ5676) DOT1L Inhibitor has clearly shown biological action, but there are still some problems to solve. Patients may find the lengthy infusion schedules inconvenient, and the exact mechanisms of resistance are still a mystery. By maintaining dormant clones or utilizing compensatory epigenetic processes, certain leukemic cells are able to adapt.
Combination therapy, biomarker development, and the creation of oral formulations with enhanced pharmacokinetics are the main areas of current research aimed at overcoming these constraints. Improved selectivity and potency are also goals of the engineering of next-generation DOT1L inhibitors. Future epigenetic modulators are being developed using the knowledge obtained from the Pinometostat (EPZ5676) DOT1L Inhibitor.
